Our News
Sodium dichloroisocyanurate
A promising candidate for the disinfection of resilient drain biofilm

Background
Biofilms are complex multicellular communities of microorganisms embedded within a protective matrix which confers resistance to various antimicrobials, including biocides. Biofilms can cause a range of human diseases and are responsible for 1.7 million hospital-acquired infections in the US annually, providing an economic burden of $11.5 billion in treatment costs. Biofilm contained within drain and plumbing systems may contain pathogenic viruses and bacteria which pose a significant risk to patient safety within healthcare environments.
Aim
The aim of this study was to determine if three hospital-grade disinfectants (sodium dichloroisocyanurate, peracetic acid and sodium hypochlorite) were capable of killing microorganisms within biofilm, and thus, determining their potential as candidates for drain biofilm disinfection.
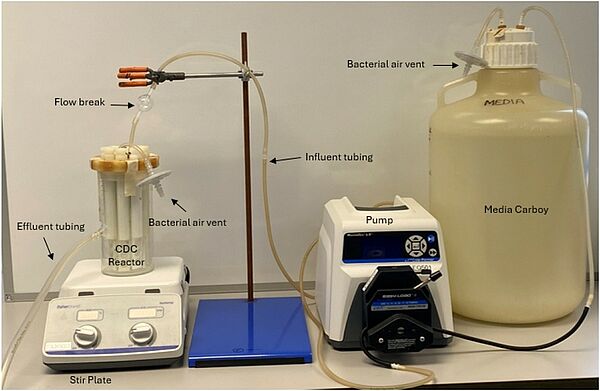
CDC Biofilm Reactor
Methods
Pseudomonas aeruginosa biofilms were cultivated using the CDC biofilm reactor, a standardised method for determining disinfectant efficacy against biofilm within the United States of America. Each disinfectant was tested using a one-minute contact time, using the highest concentration available on the product label.
Findings
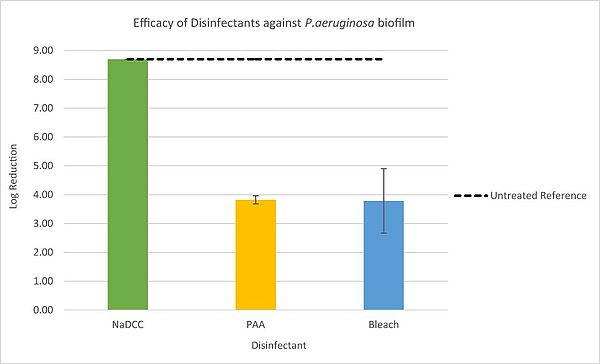
The sodium dichloroisocyanurate product was successful in killing biofilm microorganisms, resulting in a log reduction of ≥ 8.70. Peracetic acid reduced biofilm by 3.82 log10 units, followed by sodium hypochlorite, which produced a reduction of 3.78 log10 units.
Efficacy of Disinfectants against Pseudomonas aeruginosa
Conclusion
The use of a highly effective disinfectant with proven biofilm efficacy can help ensure patient safety and reduce infection levels. Drains and plumbing systems provide a reservoir for potential pathogens and biofilm; thus, drain disinfection is critical in reducing the instance of hospital-acquired infections. Sodium dichloroisocyanurate may provide a reliable solution for drain applications and subsequently, patient wellbeing and safety.
Preventable Healthcare Infections
Still Claim Lives

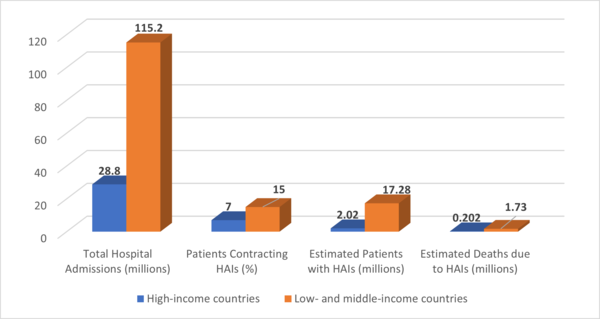
In 2022, the World Health Organization (WHO) highlighted the ongoing challenge of healthcare-associated infections (HAIs), revealing that 7 out of every 100 patients in high-income countries and 15 out of every 100 patients in low- and middle-income countries contracted an HAI during their hospital stay. Among these affected patients, approximately 10% succumbed to the infection, highlighting the critical need for better infection prevention and control (IPC) strategies globally.
The report also stressed the particular vulnerability of patients in intensive care units (ICUs) and newborns. In ICUs, up to 25% of sepsis cases are associated with healthcare interventions, and nearly half of all patients with sepsis-related organ dysfunction in these settings are battling infections acquired in the hospital.
While infection control programs have been proven to reduce the incidence of HAIs by up to 70%, many countries still face significant barriers, particularly in resource-limited settings. The COVID-19 pandemic further exposed gaps in IPC measures, pushing the urgency for global improvement in hospital hygiene practices and infection monitoring. Infections not only lead to severe health complications but also result in increased antibiotic resistance, longer hospital stays, and higher healthcare costs.
Healthcare-associated infections (HAIs) remain a critical public health issue globally, particularly in low- and middle-income countries where the burden is significantly higher than in wealthier nations. According to the World Health Organization (WHO), HAIs affect hundreds of millions of people each year and are the most frequent adverse event in healthcare settings, despite their preventability. However, accurate global statistics on HAIs are difficult to collect, as many countries lack consistent surveillance systems.

In 2023, tens of thousands of deaths worldwide were directly attributed to HAIs, with many more contributing to increased mortality through prolonged hospital stays and complications from resistant infections. Low-income countries bore a disproportionate share of this burden, with rates of infection two to three times higher than in high-income regions. This disparity is driven by factors like limited infection control resources, suboptimal hygiene practices, and the prevalence of antibiotic-resistant pathogens
The consequences of HAIs are far-reaching: they not only lead to unnecessary deaths but also result in long-term disabilities, increased antimicrobial resistance, and higher healthcare costs. WHO emphasizes that the effective implementation of infection prevention and control (IPC) measures is key to addressing this global health challenge. Despite progress in some areas, many facilities worldwide still struggle to enforce these guidelines uniformly.
In the coming years, the WHO aim to address these gaps through more robust data collection, improved global coordination, and enhanced prevention strategies, particularly in resource-constrained settings.
Incorporating Kersia NaDCC infection prevention products into hospital protocols can significantly bolster efforts to reduce healthcare-associated infections (HAIs). NaDCC, a highly effective disinfectant, offers rapid microbial action, making it a powerful tool for routine surface disinfection. By adding NaDCC to standard infection control protocols, hospitals can enhance their overall hygiene practices, ensuring that pathogens on high-touch surfaces are effectively eliminated.
This approach empowers infection preventionists by providing them with a reliable, easy-to-use solution that maintains disinfectant potency, even in the presence of organic matter. As a result, it strengthens the hospital's infection control infrastructure, reducing the incidence of HAIs and supporting a safer environment for both patients and healthcare workers
If you need further information on the benefits of Kersia NaDCC please contact:
Chris Butler: chris.butler(at)kersia-group.com
Matthew Clarke: matthew.clarke(at)kersia-group.com
References:
Transition
To NaDCC

Transitioning from sodium hypochlorite to NaDCC (sodium dichloroisocyanurate) as a primary disinfectant in a healthcare facility (HCF) involves a systematic process to ensure safety, compliance, and effectiveness. Both are powerful disinfectants, but NaDCC has certain advantages, such as a longer shelf life, better stability, and efficacy in the presence of organic matter, among others.
Four Reasons for NaDCC:
Storage and Transport Benefits: NaDCC tablets are significantly lighter and occupy less space than traditional liquid chlorine, reducing storage and transportation costs.
Extended Shelf Life: Unlike liquid chlorine, NaDCC tablets are stable with a longer shelf life, minimizing waste.
Environmental Impact: With a lower carbon footprint in production and transportation, Kersia NaDCC tablets support our goal of a more sustainable operation.
Enhanced Efficiency: NaDCC tablets are designed to achieve precise dosing, crucial in ensuring effective disinfection.

Here's how to make the transition:
Understanding the Reasons for Transition:
Begin by understanding why you're making the transition. NaDCC has several benefits over bleach, including less corrosion, better stability, improved safety profile, and effectiveness against a broad range of microorganisms.
Recognize any institutional resistance to change and prepare to address concerns through education and demonstration of NaDCC's benefits with the help of our Kersia infection prevention experts.
Regulatory Compliance and Recommendations:
Check with your local and national health regulatory body to ensure NaDCC is an approved substance for disinfection purposes in your healthcare setting.
Understand the guidelines provided by health authorities concerning the concentration, contact time, and safety precautions for NaDCC usage.

Staff Training and Education:
Arrange comprehensive training programs for your staff. These should include the reasons for the switch, the differences between bleach and NaDCC, and the proper preparation, handling, and disposal methods for NaDCC.
Highlight the correct dilution rates per tablet size; our tablets must be dissolved in water and are designed for specific concentration volumes.
Safety Protocols:
Establish safety protocols for handling and storing NaDCC, which will differ from those of bleach. Your team will notice the benefits of using NaDCC tablets straight away! They are easier to store and can be used with microfibre cloths, spray bottles, wipes and mechanical sprayers.
Create or update safety data sheets (SDS) to reflect the use of NaDCC and ensure they are readily accessible.

Disposal and Environmental Considerations:
Familiarize yourself with and adhere to local regulations on the disposal of NaDCC solutions, as requirements may differ from those for sodium hypochlorite.
Consider the environmental impact of your disinfectant and its by-products, understanding that NaDCC is typically more environmentally friendly compared to bleach.
Implementation and Monitoring:
Start with a phased approach, introducing NaDCC in one department or unit before a full-scale rollout.
Monitor and document the effectiveness of NaDCC as a disinfectant in your facility, including any impact on infection rates.
Feedback and Continuous Improvement:
Encourage staff feedback on the transition process and the use of NaDCC in everyday practice.
Continuously assess and respond to this feedback, making any necessary adjustments to protocols, concentrations, or usage guidelines.
Public Relations and Patient Communication:
Inform patients of the change in disinfection protocols, emphasizing the benefits and reassurance regarding safety and efficacy.
Prepare public relations materials, if needed, to communicate the reasons for the change and the benefits it brings to patient care and safety.
Review and Audit:
Regularly review the process, ensuring compliance with health and safety standards. Conduct regular audits to ensure the proper use and management of NaDCC, including adherence to dilution, application, and disposal procedures.
Remember, your healthcare facility is unique, so it's crucial to tailor the transition plan to meet the specific needs, concerns, and existing protocols of your institution. Always adhere to the highest standard of care, safety, and regulatory compliance.

Contact our team for more information!
Salmonella Senftenberg
What is Salmonella Senftenberg
A recent multi-country outbreak of Salmonella Senftenberg ST14 has been confirmed through a joint ECDC-EFSA assessment as “possibly linked to cherry-like tomatoes”. 92 cases of this specific serotype have been reported in 13 countries from August 2022 to July 2023. (1) Salmonella Senftenberg is a serotype of Salmonella, a genus of bacteria that's particularly notable for its association with foodborne illnesses in humans. Salmonella bacteria are rod-shaped, gram-negative, and facultatively anaerobic, which means they can grow with or without oxygen.
Salmonella Senftenberg is not one of the most common serotypes causing human disease, like Salmonella Typhimurium or Salmonella Enteritidis, but it can nonetheless cause serious illness. Salmonella lives in our intestines; an infection can be contracted by eating contaminated food or drinking contaminated water. Or by touching infected animals, their faeces or their environment.
Symptoms
Its symptoms are similar to those caused by other Salmonella strains, including fever, abdominal cramps, and diarrhoea, typically starting within 12-36 hours after infection.
Antibiotic treatment may be recommended for
People with severe illness
People with a weakened immune system, such as from HIV infection or chemotherapy treatment
Adults older than 50 who have medical problems, such as heart disease
Infants (children younger than 12 months).
Adults age 65 or older (2)
Most people recover from salmonella within four to seven days. There are some who, when the infection has ended, experience pain in their joints; this is called reactive arthritis and can last months or years and can be quite difficult to treat.
Environment
Like other serotypes, Salmonella Senftenberg can be found in a wide range of environments and hosts. It is commonly associated with the contamination of a variety of foods, including poultry, beef, pork, eggs, milk, fruits, and vegetables.
While many strains of Salmonella are highly susceptible to heat and can be killed by proper cooking methods, there are reports of certain strains exhibiting unusually high resistance to heat compared to other serotypes, which could pose challenges for food safety. (3)
Salmonella, including the serotype Senftenberg, can contaminate various fresh produce, including tomatoes, during the stages of growth, harvest, processing, or distribution. Contamination can occur in many ways, such as through contact with contaminated water, soil, and faecal matter or by cross-contamination during handling and processing. In the case of tomatoes, Salmonella can enter the fruit through cracks in the skin, the stem scar (the site where the fruit was attached to the plant), or other damage points. Certain conditions, like moisture and warm temperatures, can promote the survival and growth of Salmonella on the surface of tomatoes. There is also some evidence that Salmonella can invade the internal tissues of the plant, including the fruit, through the roots.

The conditions under which tomatoes are grown, harvested, sorted, packed, stored, and transported can all influence the risk of Salmonella contamination. Prevention measures include good agricultural practices, good manufacturing practices, proper sanitation procedures, and temperature control during storage and transport. In terms of consumer safety, it's important to wash tomatoes thoroughly before eating and refrigerate them promptly to reduce the potential for bacterial growth. For individuals with weakened immune systems, the elderly, young children, and pregnant women who are at higher risk for severe infections, it may be safest to avoid eating raw tomatoes and other fresh produce that has not been cooked.
The five keys to Safer Food are:
Keep clean
Separate raw and cooked food
Cook thoroughly
Keep food at safe temperatures
Use safe water and raw materials. (4)
Strict infection prevention protocols are crucial in the food sector to prevent outbreaks of foodborne illnesses, including those caused by Salmonella Senftenberg. This is especially true of salmonella serotypes that are potentially high heat resistance. Here are several reasons why such protocols are so important:
The food sector often caters to large numbers of people, so a single case of food contamination has the potential to cause a widespread outbreak. This is especially the case with buffet-style service, where many people can be exposed to the same contaminated food. Strict infection prevention protocols should always be in place in this situation:
Supply Chain Management: Ensuring suppliers follow good agricultural and manufacturing
Food Handling: Correct food handling practices, including proper hand hygiene among staff, preventing cross-contamination, and ensuring food is cooked to the right temperature.
Sanitation: Regular cleaning and sanitation of kitchen surfaces, utensils, and equipment to eliminate bacteria.
Food Storage: Proper food storage practices include storing foods at the correct temperatures and separating raw and cooked foods to prevent cross-contamination.
Staff Training: Regular training and education for staff on food safety practices, including the importance of staying home when sick.

We are here to help your team understand the importance of effective infection prevention. Contact one of our team to schedule a training session on our NaDCC effervescent disinfection tablets that are not only used throughout the food and hospitality sector; they are also trusted by infection preventionists in hospitals throughout the world to protect their staff, patients and visitors from pathogens like C.diff and C.auris.
Ref:
(2) www.cdc.gov/salmonella/general/index.html
(3) www.journalofdairyscience.org/article/S0022-0302(20)30442-2/pdf.
(4) www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal)/

Biofilm:
The Infection Threat Many Have Never Heard Of
In this special HAI (Healthcare Associated Infection) podcast hosted by Tyler Kern, George Clarke, founder and CEO of the UMF Corporation discuss how the COVID-19 pandemic has caused a crash course in infection prevention protocols.
Listen as they deep dive into the standard infection protection protocols established prior to COVID-19 and the updated infection prevention procedures to combat the new infection threat that many of you have yet to hear about - BIOFILM
BIOFILM – is a naturally occurring complex matrix, which can include bacteria, viruses and fungi. Biofilms allow pathogens to adhere to a solid surface and be enveloped and protected. The biofilm protects microorganisms from many disinfectant products, which means, it is essential to use the correct infection prevention disinfectant that is EPA proven effective against biofilm.
Learn about the critical importance of environmental hygiene that could reduce hospital-associated infections that exceeded 1 million infections since the 1990s with an estimated 100,000 deaths every year.
Key Takeaway Points:
• The world of infection prevention changed during the pandemic.
• Hospitals had to rethink their strategy on how to combat infections.
• Pre-Covid, healthcare admin worried about C.diff, Staph, and MRSA
Did you know that our NaDCC disinfection tablets are approved by the US EPA as effective against biofilm? In 2018 The US EPA approved Klorkleen 2 (71847-7), the first hospital grade disinfectant product to be effective against these biofilms.
Ebola
Protection & Procedures during an outbreak
Ebola Virus Disease (EVD) is a rare and deadly zoonotic disease that affects humans, monkeys, gorillas and chimpanzees It was discovered in 1976 and occasionally infects people leading to outbreaks that can spread through direct contact with bodily fluids of an infected person or objects that are contaminated by them.
This spillover event happens when a human is initially infected through contact with an infected animal such as a fruit bat or non human primate. The virus can thereafter spread from person to person, with the potential to affect large numbers of people.
Key Fact:
A person can only spread Ebola to other people after they develop signs and symptoms of Ebola. Check out the app that estimates when a person with Ebola was exposed to the virus.
www.cdc.gov/vhf/ebola/transmission/index.html
Ebola Fact: You can’t get Ebola through air / Water or Food
HOW DOES EBOLA SPREAD?
When initial exposure to the virus occurs, people do not immediately develop symptoms straight
away. An incubation period has been observed by scientists prior to the disease becoming active and when a person is contagious. Only when a person develops signs and symptoms of Ebola, can they spread the virus to others. The symptoms are headache, joint and muscle aches, weakness, diarrhoea, vomiting, rash, red eyes, stomach pain, lack of appetite, and in some cases, bleeding. The virus spreads through direct contact with broken skin, through mucous membranes in the eyes nose and mouth. Examples of direct contamination are:
• Blood or bodily fluids (saliva, sweat, urine, faeces, breast milk amniotic fluid) of an infected person or someone who has died from EVD.
• Infected fruit bats or non-human primates.
• EVD can remain in certain body fluids of a patient who has recovered, even when they no longer have symptoms.
For key facts from the World Health Organisation on Ebola virus disease click here: www.who.int/news-room/fact-sheets/detail/ebola-virus-disease
“Ebola threatens everything that makes us human” WHO
DID YOU KNOW?
3-4% of infected patients are health Care Workers!
During an outbreak, healthcare facilities should maintain a log of all persons entering the facility. Consistent standard practices are essential when caring for infected patients, healthcare
workers should:
• Wear protective clothing - to include masks, gloves, gowns and eye protection.
• Double glove if heavy duty gloves not available.
REMEMBER! The use of gloves does not replace the need for cleaning your hands!
• Isolate patients with Ebola from other patients
• Avoid direct contact with bodies of those who have died from Ebola..
• When de-robing ensure exposed surfaces do not come in contact with your body. Roll gowns in on
themselves.
Healthcare providers should frequently perform hand hygiene before and after:
• All patient contact.
• Contact with potentially infectious material
• Before putting on and upon removal of PPE - to include gloves.
Find a report form the World Health Organisation on the impact of Ebola on health workforce of Guinea, Liberia and Sierra Leone here: www.who.int/publications/i/item/WHO-EVD-SDS-REPORT-2015.1
EBOLA Patient Room Infection Control
• Dedicated medical equipment ( preferably disposable, when possible) should be used for provision of patient care.
• All non-dedicated, non-disposable medical equipment used for patient care should be cleaned and disinfected with Klorkleen @1000ppm.
Find detailed information on personal protective equipment from the World Health Organisation here: www.who.int/publications/i/item/WHO-EVD-Guidance-PPE-14.1
Procedure:
First clean the room with Klorkleen @1000ppm
• Then disinfect with fresh batch of Klorkleen solution @1000ppm
• Work from area furthest from the patient first, towards the patient (or bed)
• Ensure surface being disinfected is thoroughly wet, ideally the surface should be wet for 5 minutes
• Dispose of all used paper towels and cloths by incineration where possible.
Where cloths cannot be disposed of effectively, soak in a 1000ppm solution for 1 hour before separated laundry procedure.
Standard safety work practices combined with a robust infection prevention cleaning procedure, will help to control the infection within the facility and protect healthcare workers as they strive to save the lives of their patients.

Wash Your Hands!
Click here for the correct hand washing procedure

Aquatabs WASH KIT
EPA approve
Kersia Disinfectant Tablets with Electrostatic Sprayers
Another EPA Approval For NaDCC Disinfectant Tablets
November 4th, 2021
As a global leader in infection prevention products and solutions, we are very happy to announce the approval of an electrostatic spray application claim for use with our NaDCC disinfectant tablets. This EPA (United States Environmental Protection Agency) approval has expanded the claims for KLORSEPT (EPA Reg No. 71847-6) and KLORKLEEN 2 (EPA Reg No: 71847-7) with the addition of the electrostatic spray application to the labels.
“When you use our tablets there is no need to wait before one reoccupies a room, unlike other chemistries.” Michael Gately CEO, Medentech
Our mission is clear, assist our customers to create a safe environment that protects employees and customers alike. Protection from exposure risks connected to COVID-19 and other pathogens such as Candida Auris, C. diff, including surface biofilms.
"Our US EPA approved NaDCC disinfectant tablets have been the preferred chemistry with electrostatic sprayers based on their optimal efficacy, safety, sustainability, and ease of use. Simply fill the electrostatic sprayers with tap water and drop in a tablet. When you use our tablet (s) there is no need to wait before one reoccupies a room, unlike other chemistries.” Michael Gately CEO, Medentech.

MARBURG VIRUS
WHAT IT IS AND INFECTION PREVENTION MEASURES
Marburg virus disease (MVD) is a severe, often fatal illness in humans, causing severe haemorrhagic fever. MVD is caused by the Marburg virus, an RNA virus of the filovirus family (other members include the six Ebola virus species). The virus is transmitted to people from fruit bats and spreads among humans through human-to-human transmission.
The transmission can be via direct contact (through broken skin or mucous membranes) with the blood, secretions or other bodily fluids of infected people, and also with surfaces and materials (e.g. bedding, clothing) contaminated with these fluids.
An outbreak of Marburg virus has been reported recently in Guinea.
EFFICACY OF KLORSEPT & KLORKLEEN AGAINST MARBURG VIRUS
Infection prevention measures will be key in the prevention of the spread of this virus. EN14476:2013+A1:2015 is a European test standard that evaluates the virucidal efficacy of disinfectants via testing of Poliovirus Type 1, Adenovirus Type 5 and Murine Norovirus.
In accordance with EN14476:2013+A1:2015, Annex A, Medentechs products Klorsept, Aquatabs and Klorkleen have full virucidal activity when used as surface disinfectants. Meaning they are effective against the Marburg virus.
These products are effective when used at 500 ppm when surfaces have been pre-cleaned and at 1000 ppm when used under dirty conditions. A 5 minute contact time is required.




Prioritising WASH To Save Lives
In 2019, resolution WHA72.7 on WASH (water, sanitation and hygiene) in healthcare facilities was adopted by the World Health Assembly. The aim:- by 2025, increase the number of healthcare facilities with basic WASH facilities by 80% moving to 100% by 2030 apps.who.int/gb/ebwha/pdf_files/WHA72/A72_R7-en.pdf
The reality is that one in ten healthcare facilities globally do not have sanitation services and one in four, no basic water supply. In LDCs (least developed countries ) the problem is worse, half of the facilities lack basic water with up to 60% lacking sanitation services. COVID-19 has decimated the well meaning plans by many countries to maintain their commitment to sustainable development goal 3: (Ensure healthy lives and promote health and well-being for all at all ages) and Goal 6 (Ensure availability and sustainable management of water and sanitation for all)
However, WASH is not just about sustainable goals. It is about dignity, it's about servicing the needs of patients, it's ensuring that healthcare workers feel safe while at work, it’s about saving the lives of the many women and babies who die during childbirth for lack of basic hygiene services.
- Women need clean healthcare facilities at one of the most critical times in their lives
- They want water and soap to wash and take care of their newborns
- They want an environment that is safe from infection for their baby and their own recuperation.
Buying into WASH could reduce by half the global death rate of sepsis associated healthcare cases of which there are approx 11 million a year
Financial support is critical
WASH is a “best buy” according to the OECD (Organisation for Economic Co-operation and Development) Promoting hand hygiene and investing in better hospital hygiene have the potential to pay for themselves within a year It’s time to invest in solutions that are accessible, cost effective and sustainable, WASH must be at the highest level of global budgetary requirements and remain there for universal health to prosper.
National governments, organisations, communities and the commercial sector can move affordable
WASH in healthcare forward together by supporting lasting infrastructure.
Blended finance is one way to take the risk out of funding healthcare and scale up investment in LDCs. Combining innovative, sustainable models that can be adjusted to local requirements open opportunities and supports sustainable development.

Aquatabs continue to deliver sustainable water purification systems for schools and communities, 40 million children access safe drinking water everyday in Kenya and Nigeria.
Aquatabs Year In A Box has been designed to bring low cost infection prevention to small communities and healthcare facilities while supporting social enterprises engaged in improving access to WASH services in developing economies.
With the Aquatabs Year In A Box (YIAB) mix and match system, you receive your exact requirement for the year:
All water requirements
All surface disinfection needs
All instrument disinfection needs
All handwashing requirements
Contact us today to find out how our experts can help you with personalised infection prevention training and facility needs.
Food Insecurities – A worldwide Crisis
The 2021 global report on food crises has shown the worsening of food insecurity worldwide. Of the 55 countries highlighted with food crises, 10 countries are in crisis where households have high or above average acute malnutrition and require urgent supplies.
The main factors that exacerbate food insecurity have not changed. Prolonged conflict in many areas delay emergency operations in the field, hindering supply to critically vulnerable communities. Ongoing weather extremes such as heavy rains, severe flooding and tropical storms throughout 2020 took away the livelihoods of those affected and caused ecological devastation in parts of Africa, South Asia, Central America and the Middle East. Add to the above the economic downturn following COVID-19, the worst since World War II, and we just begin to understand the nightmare that over 155 million people are experiencing. Read the full report here: www.wfp.org/publications/global-report-food-crises-2021
In the midst of all this are children who are facing severe acute malnutrition.
In 2020 15.8 million children under the age of 5 years old were suffering from wasting, half of those live in the 10 countries found to be in phase 3 or above on the integrated food security classification (IPC). Phase 3 classifies as “households experiencing food consumption gaps with high or above usual acute malnutrition / accelerated depletion of livelihood assets / resort to crisis coping strategies”
1st 1000 days
The first two years of a child’s life, also known as the first 1000 days, are critical for cognisance. Malnutrition damages a child’s cognitive and physical development, it is mostly irreversible and leads to illness, severely depletes the quality of a child’s life and leads to inequalities in adult life.
A baby’s world revolves around the love, protection and nutrition mother provides. Good maternal health is essential for a healthy baby, yet breastfeeding women are projected to continue being acutely malnourished in 2021.
“Diseases and a poor health environment are key drivers of childhood malnutrition”
WHO Director-General Dr Tedros Adhanom Ghebreyesus
Children who are malnourished are further susceptible to, and have a higher burden to debilitating water borne disease such as cholera and giardia. Limiting access to safe drinking water and RUTF (ready-to-use therapeutic food) significantly reduces their chances of survival. In the following link you will find a publication that demonstrates the impact of a WASH intervention in the management of severe acute malnutrition. In brief, the main robust conclusions were:
Point-of-use water treatment improves recovery rates among children with severe acute malnutrition in Pakistan : results from a site-randomized trial:
Recovery rates 16.7- 22.2% higher among children receiving water treatment
• The intervention decreased the time-to-recovery (4.4 days; P = 0.038),
• It improved the recovery rate (10.5%; P = 0.034),
• It increased the absolute weight gain (3.0 g/d; P = 0.014).
Find the full report here: pubmed.ncbi.nlm.nih.gov/29488461/
We are very much focused on playing our part, whether it be delivering
- SUSTAINABLE WASH water purification systems for schools and rural communities.
- Providing high quality affordable WASH’NUTRITION essential water and nutritional products.
- WASH in HEALTHCARE effective infection prevention solutions for water, surface, instruments and hands in hospitals and small communities
Contact us to find out more about our activities in creating social enterprises, bringing sustainable solutions to healthcare, making water safe and nutrition accessible in the most challenging circumstances: [email protected]


Aquatabs Year In A Box (YIAB) Low cost mix and match disinfection products for rural healthcare centres and hospitals. Aquatabs Flo and Aquatabs InLine for disinfection of large volumes of water - Klorkleen for surface disinfection - Ultraseptin for sterilisation of endoscopes and medical instruments.
Coronavirus (2019-nCoV)
WHO recommends NaDCC Granules
5th February 2020
The 2019 novel coronavirus (2019-nCoV) outbreak is continuing to evolve with many cases having now been detected outside of Wuhan, China. As of February 4th, the World Health Organisation (WHO) have reported 425 deaths in China and 20,630 confirmed cases globally. A total of 159 cases have been identified outside of China, spread worldwide across 23 countries. There has been one recorded death outside of China.
As a result, the WHO have declared the 2019-nCoV outbreak a public health emergency of international concern (PHEIC). By declaring this outbreak a PHEIC, the WHO has highlighted the urgency and need for international efforts to minimize further spread. The aim of a PHEIC is to ensure a global response that is evidence-based, measured and balanced. The enhanced cooperation will increase transparency, the development of vaccines, diagnostics and therapeutics. For more information on the WHO’s response, please visit:
Although the WHO is still to determine the survival capabilities of 2019-nCoV on surfaces, preliminary information suggests the virus could survive for several hours. In the case of high touch surfaces which could harbour respiratory droplets from an infected patient, regular disinfection is necessary. Our surface disinfectant products are effective at killing this viral pathogen. The WHO have also recommended the use of NaDCC granules for the disinfection of healthcare facilities as part of their recommended Disease commodity package – Novel Coronavirus
www.who.int/publications-detail/disease-commodity-package---novel-coronavirus-(ncov)
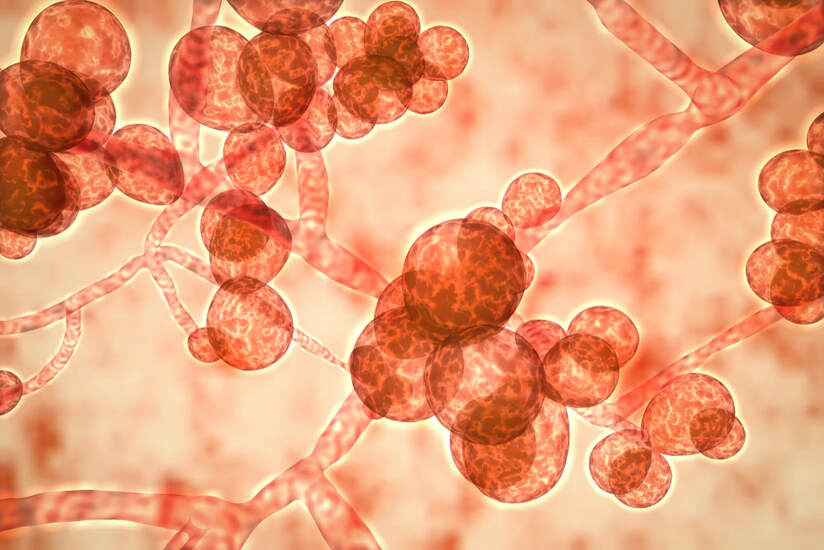
Candida auris
An Unusual Yeast Causing Serious Infection
Candida auris – An Unusual Yeast Causing Serious Infection
Over the past number of years discussion surrounding Candida auris has continued to increase. This pathogen is regularly discussed by the media and governmental bodies. It is of high concern to healthcare facilities. But what is Candida auris and why is it gaining so much attention?
Candida auris is a fungal pathogen, to be more specific, it is a type of yeast. This pathogen was first reported in Japan in 2009 and has since been linked with healthcare associated infections (HAIs) on five continents (1). C. auris control is challenging due to the difficulty in identifying this pathogen in the clinical laboratory. Moreover, C. auris often exhibits high levels of multi drug resistance, leading to high rates of treatment failure with invasive infections (2). The United States Centers for Disease Control and Prevention (CDC) map new case reports worldwide (https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html).
As of January 31st, 2019, a total of 560 confirmed clinical cases have been identified in the U.S.
The most commonly observed invasive infection associated with C. auris has been blood stream infections, with mortality in the range of 30 to 60%. Patients involved generally have high healthcare exposure, however, infection may not be apparent until weeks after initial admission (2). The best treatment practice for C. auris infection has not been defined.
C. auris appears to spread via patients and the environment. It has be isolated from the skin of affected patients as well a patient contact areas, such as sinks, medical equipment, furniture and mattresses (3, 4). Recent investigations suggest quaternary ammonium compounds may not be effective against C. auris (5). The US CDC recommend that sporicidal disinfectants (those listed on the U.S. EPA List K) be used where C. auris is of concern and provide several other points of interest in relation to C. auris transmission control, such as hand hygiene and contact precautions (https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html).
There is still a lot to learn regarding this pathogen, such as its origin in the natural environment, the reason for its sudden emergence and high antifungal resistance. Healthcare workers should remain ever vigilant for C. auris where Candida spp. infection is suspected due to its impact on patient mortality. Undoubtedly control of these outbreaks will prove a challenge until more information about this unusual pathogen become available. Providing care in accordance with best practice outlined by the CDC will be key until more defined control mechanisms become apparent.
1. Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. 2009. Candida auris sp . nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol: 53:41–44
2. Spivak ES, Hanson E. 2018. Candida auris : an Emerging Fungal Pathogen. J. Clin. Microbiol. 56:1–10.
3. Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J,Palmore TN, Zelzany A, Adams EH,Quinn M, Chaturvedi, S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J,Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2017. Investigation of the First Seven Reported Cases of Candida auris , a Globally Emerging Invasive Multidrug-Resistant Fungus — United States , May 2013 – August 2016. Amer. J. Transplant. 17:296-299
4.Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, Ryan L, Shackleton J, Trimlett R, Meis JF, Armstrong-james D, Fisher MC. 2016. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 5:35.
5. Cadnum JL, Shaikh AA, Christina T, Sankar T, Jencson AL, Larkin EL, Ghannoum MA, Donskey CJ. 2017. Effectiveness of Disinfectants Against Candida auris and Other Candida Species. Infect. Control and Hosp. Emidemiol. 38:1–4.